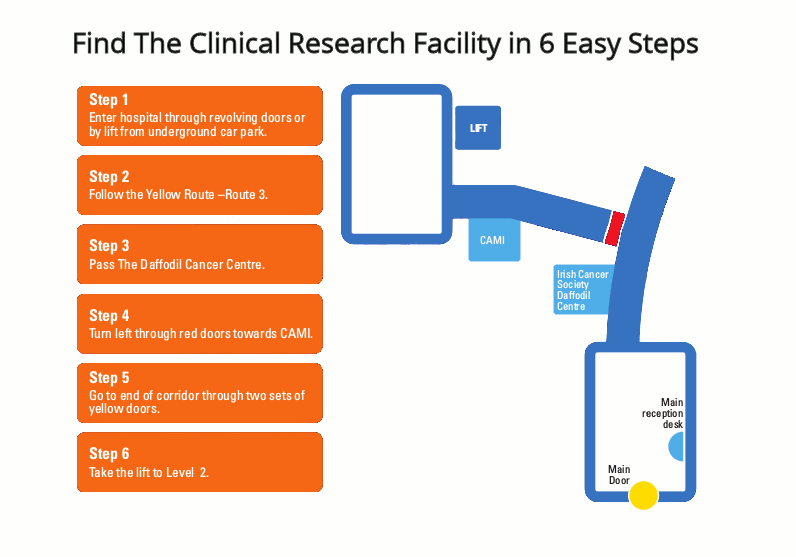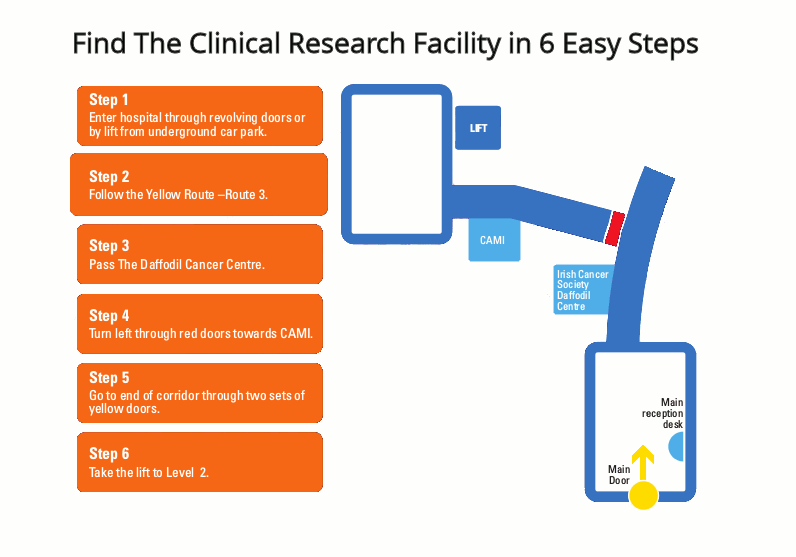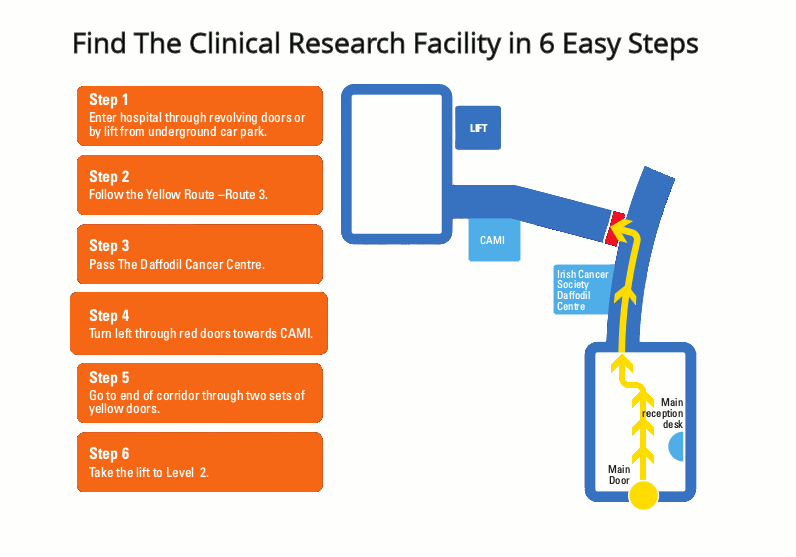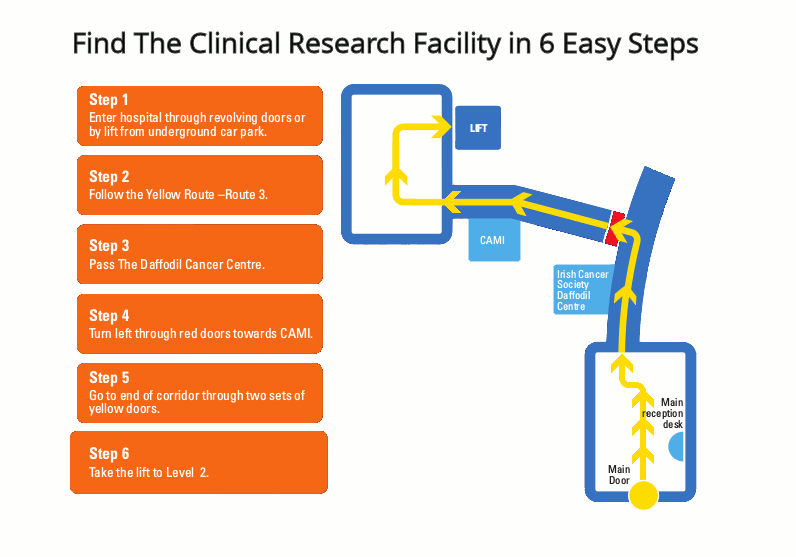
Respiratory
The Respiratory Department at St James’s hospital run a number of both investigator-led and industry centered drug trials in respiratory in conjunction with the CRF.
| Study Name | Study Title | Study Type | Sponsor | Sponsor Type |
|---|---|---|---|---|
| Insmed (ASPEN) | A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, MultiCenter Study to Assess the Efficacy, Safety, and Tolerability of INS1007 Administered Once Daily for 52 Weeks in Subjects with Non-CysticFibrosis Bronchiectasis | Clinical Trial (2020-003688-25) (NCT04594369) | Insmed | Commercial: Industry |
| GSK3511294-Nimble | A 52-week, randomised, double-blind, double-dummy, parallel group, multicentre, non-inferiority study assessing exacerbation rate, additional measures of asthma control and safety in adult and adolescent severe asthmatic participants with an eosinophilic phenotype treated with GSK3511294 compared with mepolizumab or benralizumab | Clinical trial (NCT04718389) | GSK | Commercial: Industry |
| BAL dccr | Airway Gene Expression Profiling and Immune Responses to Lung Disease (BAL) | Observational-non invasive | N/A | Non-commercial: Academic |
| Study Name | Study Title | Study Type | Sponsor | Sponsor Type |
|---|---|---|---|---|
| Thoracic Biobank | Estbalishment of a Thoracic Biobank to use in molecular and Cellular Studies | Prospective Cohort | St. James's Foundation | Non-commercial: Hospital |
| MA39297 | An international trial to characterize the disease behaviour of idiopathic pulmonary fibrosis and interstitial lung disease during the peri-diagnostic period | Clinical Trial (NCT03261037) | F Hoffmann- La Roche Ltd | Commercial: Industry |
| Luster | A 52 week, multicenter, randomized, double-blind, placebo-controlled study to assess the efficacy and safety of QAW039 when added to existing asthma therapy in patients with uncontrolled severe asthma | Clinical Trial (NCT02555683) (2015-002553-35) | Novartis Pharma UK Ltd | Commercial: Industry |
| GALACTIC-1 | A randomized, double-blind, multicentre, parallel, placebo-controlled study in patients with idiopathic pulmonary fibrosis (IPF) investigating the safety and efficacy of TD139, a dry powder, inhaled galectin-3 inhibitor | Clinical Trial (NCT03832946) (2018-002664-73) | Galecto Biotech AB | Industry |
| Crystal | Crystal - A prospective, multicentre, 12 week, randomized open label study to evaluate the efficacy and safety of glycopyrronium (50 microgrms o.d.) or indacaterol maleate and glycopyrronium bromide fixed dose combination (110/50 micrograms o.d.) regarding symptoms and health status in patients with moderate chronic obstructive pulmonary disease (COPD) switching from treatment with any standard COPD regimen | Clinical trial (NCT01985334) | Novartis | Industry |
| Palladium | A multi-centre, randomised, 52 week treatment, double-blind, triple-dummy, parallel-group study to assess the efficacy and safety of QMF149 compared with mometason furoate in patients with asthma | Clinical trial (NCT02554786) | Novartis Pharma UK Ltd | Industry |
| IPF/Sarcoidosis | IPF/Sarcoidosis (DCCR) | Observational with radiology or blood tests | DCCR | Non-commercial: Academic |
| IGRA | IGRA (DCCR) | Observational with radiology or blood tests | HSE - Health Protection Surveillance Centre | Non-commercial: Academic |
| INCA | Inhaler Compliance in Asthma (INCA) | Medical device study (NCT01529697) | Health Research Board | Non-commercial: Academic |